QUANTITATIVE PROTEOMICS OF PLASMA EXTRACELLULAR VESICLES REVEALS A TTR - PLASMINOGEN NETWORK IN ATTR CARDIAC AMYLOIDOSIS
QUANTITATIVE PROTEOMICS OF PLASMA EXTRACELLULAR VESICLES REVEALS A TTR - PLASMINOGEN NETWORK IN ATTR CARDIAC AMYLOIDOSIS
Zaroui, A.; Habert, D.; Vallee, B.; Kharoubi, M.; Fellahi, S.; Vingert, B.; Seve, M.; Cascone, I.; Audard, V.; Itti, E.; Abroud, H.; Galat, A.; Damy, T.; Bourgoin-Voillard, S.; Molinier-Frenkel, V.
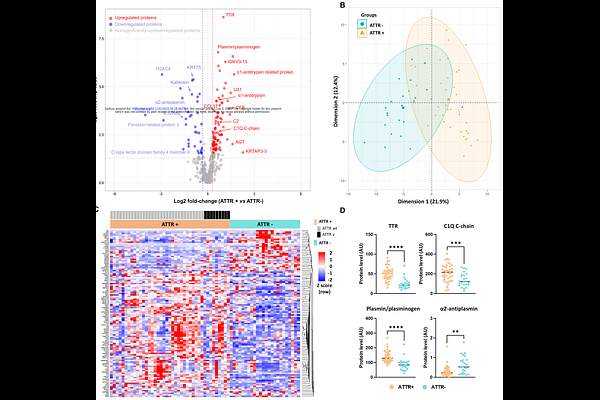
AbstractBackground: Despite recent progress, the prognosis of patients with transthyretin (TTR) cardiac amyloidosis remains poor; this is primarily due to late diagnosis, when irreversible damage has already occurred. Today\'s diagnostic work-up still relies on peripheral tissue or a cardiac biopsy, while circulating levels of TTR or other plasma markers have little diagnostic value. Although extracellular vesicles (EVs, as key mediators of intercellular communication) may reflect disease-specific molecular changes, their protein cargo has not yet been explored in the context of TTR amyloidosis (ATTR) cardiomyopathy. Objectives: To characterize the plasma EV proteome in ATTR cardiomyopathy and identify potential biomarkers for pathophysiological pathways, diagnosis, or prognosis. Methods: We performed mass-spectrometry-based, label-free, proteomic profiling of plasma EVs from 65 patients with hypertrophic cardiomyopathy due to TTR amyloidosis (the ATTR+ group, n=41) or non-amyloid cardiac disease (the ATTR- group, n=24). The groups were matched by age and sex. Results: A distinct protein signature comprising 117 deregulated proteins was identified in EVs from ATTR+ patients. The ATTR+ EVs were enriched in proteins associated with vascular homeostasis, coagulation, and inflammation. At least 18 of these proteins formed an interconnected network centered on plasmin/plasminogen. Notably, EV levels of TTR and plasminogen levels were elevated, while the level of alpha2-antiplasmin (plasmin\'s primary inhibitor) was low. This imbalance is particularly relevant because plasmin is known to promote amyloidogenesis via TTR cleavage. Conclusions: Our findings provide new insights into the molecular mechanisms underlying ATTR cardiomyopathy and suggest that plasma EV proteins are potential diagnostic or prognostic biomarkers and/or therapeutic targets.