LSH-mediated resolution of R-loops mitigates transcription-replication conflicts to preserve genomic stability in prostate cancer cells
LSH-mediated resolution of R-loops mitigates transcription-replication conflicts to preserve genomic stability in prostate cancer cells
Ni, K.; Zhang, Y.; Zheng, K.; Huang, J.; Xiong, N.; Liang, R.; Chen, Y.; Jin, H.; Hai, Y.; Li, W.; Ma, J.; Yang, R.; Xia, G.; Bai, Y.; Song, L.; Hu, X.; Tang, Z.; Fu, Q.
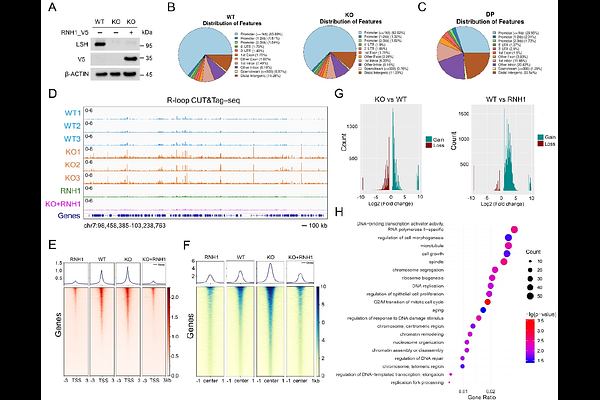
AbstractProstate cancer cells exhibit heightened transcriptional activity to sustain their aggressive phenotype, yet this creates DNA topological stress, leading to RNA:DNA hybrids (R-loops) and DNA damage that compromise genomic stability. LSH, an SNF2-family chromatin remodeler, is frequently dysregulated in cancers and linked to tumor progression, but its mechanistic role in genome maintenance remains unclear. Here, we demonstrate that LSH resolves R-loops in an ATP-dependent manner, mitigating transcription-replication (TR) conflicts in prostate cancer cells. LSH depletion caused R-loop accumulation due to reduced FANCD2 recruitment, exacerbating DNA damage and impairing Rad51 filament formation. Strikingly, LSH deficiency also increased R-loops at promoters of MYC and E2F target genes, stalling RNA polymerase II elongation and disrupting transcriptional programs critical for proliferation. Our findings reveal LSH is a key guardian of genome integrity, resolving R-loops to prevent TR conflicts and maintaining DNA repair fidelity. By linking LSH to R-loop resolution and transcriptional regulation, this study reveals its dual role in prostate cancer pathogenesis-supporting malignant proliferation while safeguarding genomic stability. These insights nominate LSH as a potential therapeutic target to disrupt oncogenic transcription and induce synthetic lethality in prostate cancer.