R2HaPpY: Rapid-robust phosphotyrosine peptide enrichment using HaloTag-Src SH2 pY superbinder
R2HaPpY: Rapid-robust phosphotyrosine peptide enrichment using HaloTag-Src SH2 pY superbinder
Chang, A.; Rodriguez-Mias, R. A.; Berg, M. D.; Moggridge, S.; Villen, J.
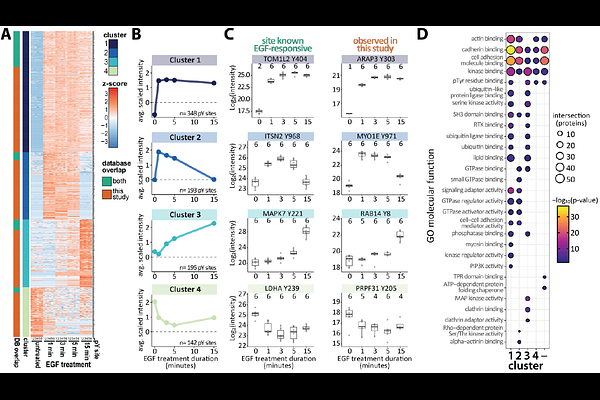
AbstractPhosphotyrosine signaling plays a critical role in many biological processes, from cell proliferation to immune response. Despite its importance, systems-level analysis of phosphotyrosine signaling remains a challenge due to costly enrichment reagents and labor-intensive protocols. We previously established an automated phosphotyrosine enrichment method for preparing 96 samples in parallel. Here, we further optimize this method by fusing an SH2 phosphotyrosine superbinder to the HaloTag protein. This allows simple and cost-effective preparation of enrichment beads directly from bacterial lysate, expediting reagent preparation from days to hours. Additionally, our new reagent binds phosphotyrosine peptides at higher efficiency than other enrichment reagents. Using this reagent, we detect and quantify 1,651 unique phosphotyrosine sites from EGF stimulated HeLa cells using only ~1 mg of input peptides per replicate. These include 878 regulated pY sites, many of which are low abundance and not previously detected or annotated as EGF-responsive. This streamlined and sensitive method facilitates comprehensive, quantitative mapping of tyrosine phosphorylation dynamics, enabling broader integration of phosphotyrosine signaling into multiomic and network-level models across diverse biological systems and disease states.