Disease-causing mutations in the G protein β5 β-propeller disrupt its chaperonin-mediated folding trajectory
Disease-causing mutations in the G protein β5 β-propeller disrupt its chaperonin-mediated folding trajectory
Sass, M. I.; Mack, D. C.; Nickles, R. A.; Jones, C. A.; Brunsdale, R. L.; Cottam, S. L.; Shen, P. S.; Willardson, B. M.
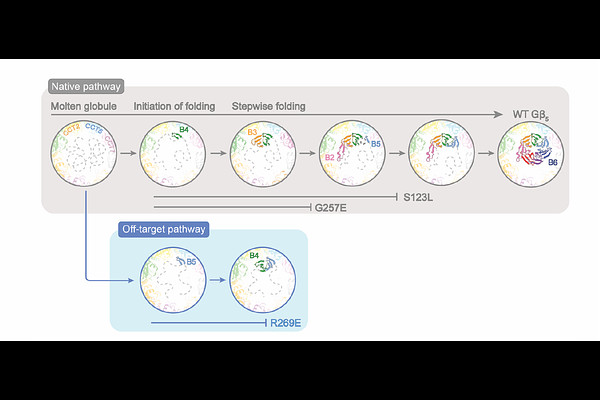
AbstractThe Chaperonin Containing Tailless polypeptide 1 (CCT or TRiC) is an essential cytosolic chaperone that folds multiple protein substrates, including many with {beta}-propeller folds. One {beta}-propeller substrate is the G protein {beta}5 subunit (G{beta}5) of Regulator of G protein Signaling (RGS) complexes that determine the duration of G protein signals in neurons. In recent work, we used cryo-electron microscopy (cryo-EM) to visualize the complete CCT-mediated folding trajectory for G{beta}5, from an initiating electrostatic interaction of a single {beta}-strand in G{beta}5 with CCT5 to a completely folded {beta}-propeller structure. Here, we used biochemistry and cryo-EM to determine how missense mutations in G{beta}5, including those that cause severe neurological diseases, alter the G{beta}5 folding trajectory and lead to incompletely folded, trapped intermediates. These findings highlight how defects in chaperonin-mediated folding contribute to disease and suggest potential strategies for stabilizing misfolded proteins to restore function.