Immunoblot-based activity assay for heme-containing histidine kinases
Immunoblot-based activity assay for heme-containing histidine kinases
Larson, G.; Bhagi-Damodaran, A.; Rama Damodaran, A.
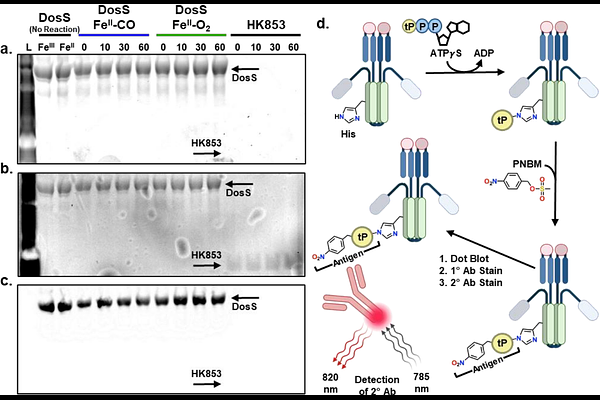
AbstractHistidine kinases (HKs) are essential bacterial signal transduction proteins and attractive drug targets due to their critical cellular functions. Direct measurement of their autophosphorylation activity is crucial for developing inhibitors and advancing disease treatments. While [{gamma}-32P]-ATP radiolabeling has long been a conventional method for kinase activity measurements, its reliance on 32P introduces inherent limitations. The short half-life of this isotope imposes time-sensitive constraints on experiments, and stringent radiation safety and compliance requirements significantly increase operational costs and hinder scalability. Several alternative methods, including fluorescent phosphate-binding probes and fluorescence- or luminescence-based antibodies, have been developed for HKs that overcome these challenges. However, for heme-based HKs, these alternatives suffer from interference due to intrinsic heme fluorescence and luminescence. In this work, we address this interference by employing a near-IR fluorophore-labeled secondary antibody. Combined with an ATP{gamma}S immunoblot-based method, we demonstrate semi-quantitative detection of thiophosphorylation activities of Mycobacterium tuberculosis DosS (a prototypical heme-based HK) in different ligation states. Consistent with previous radiolabeling studies, we show that the ability of DosS to auto-thiophosphorylate is significantly enhanced with CO as compared to O2. This low-cost, easy-to-implement method simplifies and democratizes heme-based HK activity measurement.