Synaptic Spine Head Morphodynamics from Graph Grammar Rules for Actin Dynamics
Synaptic Spine Head Morphodynamics from Graph Grammar Rules for Actin Dynamics
Hur, M.; Bartol, T.; Rangamani, P.; Sejnowski, T.; Mjolsness, E.
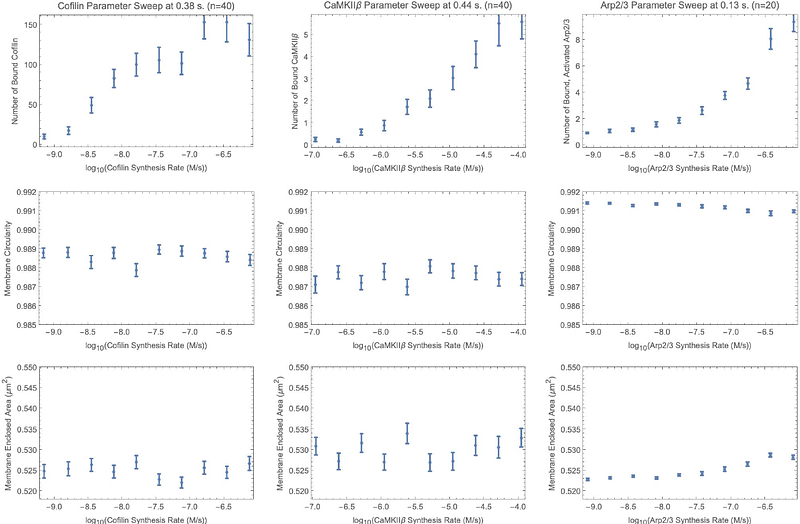
AbstractThere is a morphodynamic component to synaptic learning by which changes in dendritic spine head size are associated with the strengthening or weakening of the synaptic connection between two neurons, in response to the temporal correlation of local presynaptic and postsynaptic signals. Morphological factors are in turn sculpted by the dynamics of the actin cytoskeleton. We use Dynamical Graph Grammars (DGGs) implemented within a computer algebra system to model how networks of actin filaments can dynamically grow or shrink, reshaping the spine head. DGGs provide a well-defined way to accommodate dynamically changing system structure such as active cytoskeleton represented using dynamic graphs, within nonequilibrium statistical physics under the master equation. We show that DGGs can also incorporate biophysical forces between graph-connected objects at a finer time scale, with specialized DGG kinetic rules obeying biophysical constraints of Galilean invariance, conservation of momentum, and dissipation of conserved global energy. We use graph-local energy functions for cytoskeleton networks interacting with membranes, and derive DGG rules from the specialization of dissipative stochastic dynamics to a mutually exclusive and exhaustive collection of graph-local neighborhood types for the rule left hand sides. Dissipative rules comprise a stochastic version of gradient descent dynamics. Thermal noise rules use a Gaussian approximation of each position coordinate to sample jitter-like displacements. We designed and implemented DGG grammar sub-models including actin network growth, non-equilibrium statistical mechanics, and filament-membrane mechanical interaction to regulate the re-writing of graph objects. From a biological perspective, we observe regulatory effects of three actin-binding proteins on the membrane size and find evidence supporting mechanisms of membrane growth.