Resources for molecular studies of unculturable obligate biotrophic fungal plant pathogens using their saprotrophic relatives
Resources for molecular studies of unculturable obligate biotrophic fungal plant pathogens using their saprotrophic relatives
Loos, A.; Doykova, E.; Qian, J.; Kümmel, F.; Ibrahim, H.; Kiss, L.; Panstruga, R.; Kusch, S.
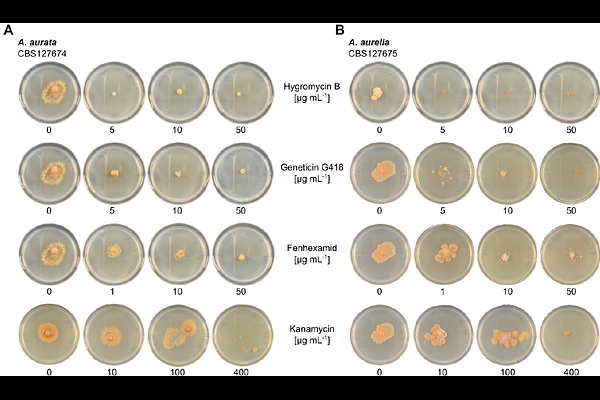
AbstractObligate biotrophic plant pathogens like the powdery mildew fungi commit to a closely dependent relationship with their plant hosts and have lost the ability to grow and reproduce independently. Thus, at present, these organisms are not amenable to in vitro cultivation, which is a prerequisite for effective genetic modification and functional molecular studies. Saprotrophic fungi of the family Arachnopezizaceae are the closest known extant relatives of the powdery mildew fungi and may hold great potential for studying genetic components of their obligate biotrophic lifestyle. Here, we established telomere-to-telomere genome assemblies for two representatives of this family, Arachnopeziza aurata and A. aurelia. Both species harbor haploid genomes that are composed of 16 chromosomes at a genome size of 43.1 and 46.3 million base-pairs, respectively, which, in contrast to most powdery mildew genomes that are transposon-enriched, show a repeat content below 5% and signs of repeat-induced point mutation (RIP). Both species could be grown in liquid culture and on solid standard media and were sensitive to common fungicides such as hygromycin and fenhexamid. We successfully expressed a red fluorescent protein and hygromycin resistance in A. aurata following polyethylene glycol-mediated protoplast transformation, demonstrating that Arachnopeziza species are amenable to genetic alterations that may include gene replacement, gene modification, and gene complementation. With this work, we established a potential model system that promises to sidestep the need for genetic modification of powdery mildew fungi by using Arachnopeziza species as a proxy to uncover the molecular functions of powdery mildew proteins.