Epithelial-Immune-Stromal Interactions Define Divergent Repair and Fibrosis Pathways After Acute Kidney Injury in Human Renal Transplants
Epithelial-Immune-Stromal Interactions Define Divergent Repair and Fibrosis Pathways After Acute Kidney Injury in Human Renal Transplants
Azim, S.; Rousselle, T.; Zubair, H.; Shetty, A. C.; Archer, K. J.; Marshall, J.; Rajabi, A.; Lara, C.; Mustofa, S.; Drachenberg, C.; Bromberg, J.; Menon, M.; Maluf, D. G.; Akalin, E.; Mas, V. R.
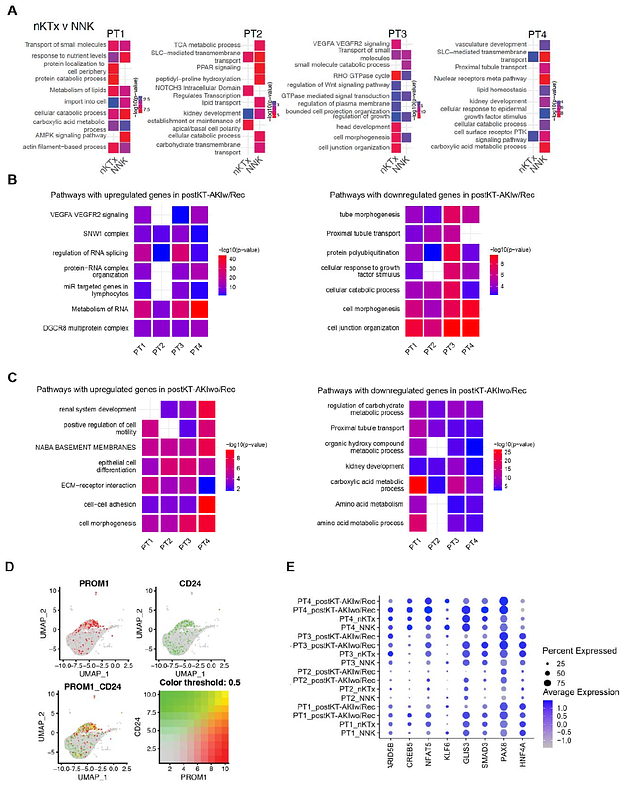
AbstractAcute kidney injury (AKI) is a major cause of early graft dysfunction after kidney transplantation, particularly in recipients of high-risk donor kidneys prone to ischemia-reperfusion injury. However, the cellular mechanisms dictating whether injury resolves or progresses to fibrosis remain unclear. This study combines single-nucleus RNA sequencing and imaging mass cytometry (IMC) analysis of human kidney allograft biopsies collected within eight weeks posttransplant, stratified by long-term functional outcomes. Grafts that recovered function were enriched in regenerative proximal tubular (PT) cells co-expressing PROM1, CD24, and injury markers, consistent with scattered tubular cells (STCs). In contrast, non-recovering grafts contained a unique subpopulation of transitional proximal tubule cells (tPT4) characterized by dedifferentiation, loss of epithelial identity, and acquisition of fibroblast-like features. Fibroblast trajectory analysis revealed a profibrotic lineage, progressing from stromal progenitors to myofibroblasts, exclusive to nonrecovery grafts. Immune profiling showed divergent macrophage (M{varphi}) polarization, with reparative M{varphi}2 cells and regulatory dendritic cell (DC)-like signatures in recovering grafts, versus inflammatory M{varphi}1 and pro-fibrotic DCs in non-recovery. IMC confirmed spatial colocalization of injured tubules, activated fibroblasts, and immune cells in fibrotic regions, validated in an independent cohort. Functional assays demonstrated that ischemic epithelial injury activated monocyte-derived M{varphi}s with mixed inflammatory/reparative profiles and induced fibroblast-related gene expression, while PAX8 knockdown impaired epithelial proliferation and promoted pro-inflammatory signaling. These findings reveal epithelial cell plasticity as a central driver of divergent repair outcomes following renal transplant AKI and highlight epithelial-immune-stromal crosstalk as a therapeutic target to promote recovery and prevent chronic graft injury.