Non-Equilibrium Snapshots of Ligand Efficacy at the μ-Opioid Receptor
Non-Equilibrium Snapshots of Ligand Efficacy at the μ-Opioid Receptor
Robertson, M. J.; Papasergi-Scott, M. M.; Peroto, M. C.; Varga, B. R.; Majumdar, S.; Skiniotis, G.
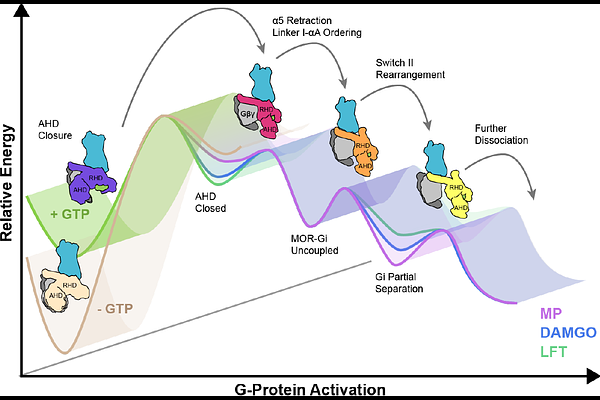
AbstractDistinct ligands for the same G-protein coupled receptor (GPCR) activate intracellular signaling partners to varying extents, but the molecular mechanisms driving these differences remains elusive. Hypothesizing that such differences in signaling efficacy may be captured structurally in intermediate states under non-equilibrium conditions, we implemented a time-resolved (TR) cryo-EM approach to visualize the GTP-induced activation of the GI{beta}{gamma} heterotrimer by the -opioid receptor (MOR) bound to three ligands displaying partial, full, or super-agonism on the receptor. We resolved ensembles of conformational states along the G-protein activation pathway, including a previously unobserved intermediate state that enabled us to visualize receptor dynamics as a function of bound ligand. The results demonstrate ligand-dependent differences in state occupancy and conformational stability, with higher ligand efficacy correlating with increased dynamics of the receptor\'s transmembrane (TM) helices 5 and 6. Furthermore, we identify key mechanistic differences in the GTP-induced activation of Gi compared to Gs that likely underlie their distinct activation kinetics. Corroborated by molecular dynamics (MD) simulations, these findings provide a dynamic structural landscape of GPCR-G-protein interactions for ligands of different efficacy and suggest partial agonists may produce a \'kinetic trap\' during G-protein activation.