The Regulation of COX-2, ABCA1 and ABCG1 by the lncRNA PACERR links the inflammatory response and cholesterol homeostasis
The Regulation of COX-2, ABCA1 and ABCG1 by the lncRNA PACERR links the inflammatory response and cholesterol homeostasis
Hennessy, E. J.; Tanosaki, T.; Lordan, R. J.; Tang, S. Y.; Shuvaev, V.; Ghosh, S.; Das, U.; Joshi, R.; Meng, H.; Snouwaert, J. n.; Winter, H.; Maegdefessel, L.; Koller, B. N.; FitzGerald, G. A.
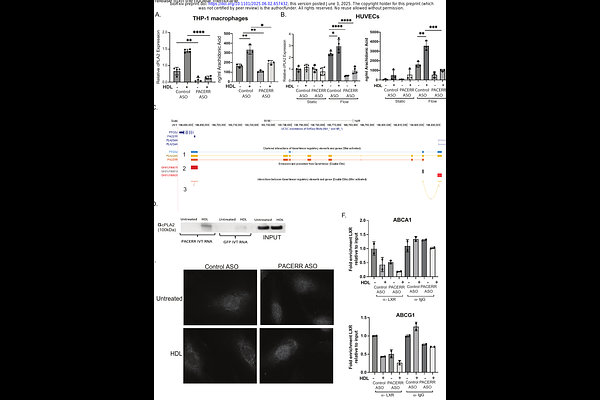
AbstractCyclooxygenase-2 (COX-2) has an established role in inflammation and its inhibition by non-steroidal anti-inflammatory drugs (NSAIDs) is clinically efficacious1,2. However, placebo-controlled trials of COX-2 inhibitors have revealed a cardiovascular hazard. This has been attributed to suppression of COX-2 dependent cardioprotective prostaglandins (PGs), especially prostacyclin, produced by endothelial (EC) and vascular smooth muscle cells (vSMC) 1-3. Upstream of these effects, we have found that the COX-2 antisense long non-coding RNA (lncRNA) PACERR interacts with discrete RNA binding proteins to mediate the cell specific COX-2 response to both anti- [high-density lipoprotein (HDL)] and pro-inflammatory [lipopolysaccharide (LPS)] stimuli. PACERR exerts its action both in cis and in trans, controlling the expression of COX-2 and the cholesterol transport genes, ABCA1 and ABCG1. When cells are exposed to HDL, PACERR is transcribed, leading to the release of bound histone acetyltransferase p300 and its relocation to the promoter of COX-2 which is then transcribed leading to PG formation. In concert, PACERR also releases heterogeneous nuclear ribonucleoprotein L (hnRNPL) which relocates to the ABCA1 and ABCG1 gene loci, controlling mRNA alternative splicing and subsequent cholesterol efflux to HDL. Silencing PACERR, with an antisense oligonucleotide (ASO), augments the COX-2 and cholesterol efflux in response to HDL. In response to HDL, PACERR associates with its neighboring gene, cytosolic phospholipase A2 (cPLA2/PLA2G4A) both interacting with an enhancer element affecting cPLA2 transcriptional activation and escorting the cPLA2 protein to the nuclear membrane. There, it releases arachidonic acid, further activating COX-2 but limiting ABCA1 and ABCG1 by suppressing the formation of the LXR/RXR heterodimers required for their transcription when cholesterol efflux is no longer required 4. In response to LPS, PACERR controls the action of the NF{kappa}B repressive protein, I{kappa}B{zeta}. When PACERR is silenced in a humanized mouse model with the ASO, I{kappa}B{zeta} is free to bind to the NF{kappa}B site in the COX-2 promoter, suppressing transcription, PG formation and systemic inflammation. In summary, these studies reveal dual and complementary mechanisms by which PACERR modulates PG production by COX-2. In response to HDL, it enhances production of prostacyclin whereas it dampens production of the PG response to LPS.