Notch3 regulates pericyte phenotypic plasticity in colorectal cancer
Notch3 regulates pericyte phenotypic plasticity in colorectal cancer
Koliaraki, V.; Chalkidi, N.; Stavropoulou, A.; Arvaniti, V. Z.; Paraskeva, C.; Monogyiou, A.; Sakkou, M.; Nikolaou, C.
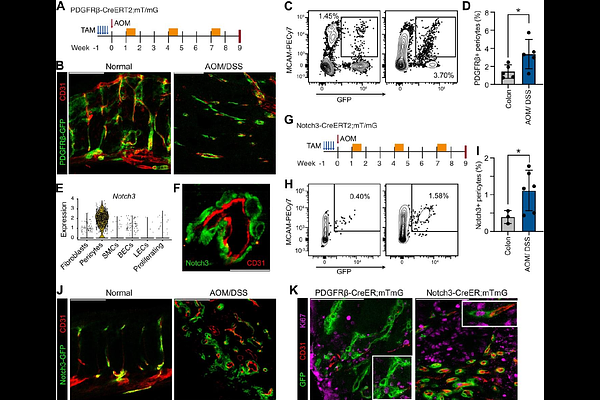
AbstractPericytes undergo phenotypic alterations that influence cancer progression, yet the molecular mechanisms governing these changes remain poorly understood. Here, we investigated the role of Notch3 signaling in pericyte phenotype and functions in colorectal cancer (CRC). Using lineage tracing approaches, we showed that tumor pericytes originate from normal tissue-resident pericytes, which proliferate inside tumors. In vivo genetic manipulation revealed that Notch3 pathway activation promotes pericyte proliferation, while suppressing contractile protein expression, and leads to increased endothelial cell proliferation. In contrast, Notch3 deletion leads to reduced endothelial proliferation and a significant decrease in. This effect is associated with a shift toward a contractile phenotype. Single-cell RNA sequencing analysis uncovered significant pericyte heterogeneity in both mouse colitis-associated cancer and human CRC. It specifically identified functionally distinct subpopulations characterized by differential Notch3 activity, which supported our in vivo and in vitro findings. Our results establish Notch3 as a key regulator of pericyte phenotypic plasticity in CRC and suggest that targeting this pathway could represent a promising strategy for improving therapeutic outcomes through vascular normalization.