Tryptophan-aspartic acid containing peptide analog from coronin 1 inhibits model membrane fusion and enveloped viral infection in cells
Tryptophan-aspartic acid containing peptide analog from coronin 1 inhibits model membrane fusion and enveloped viral infection in cells
Pandia, S.; Qazi, B.; Viswakarma, V.; Gautam, S.; Meshram, C. D.; Chakraborty, H.; Haldar, S.
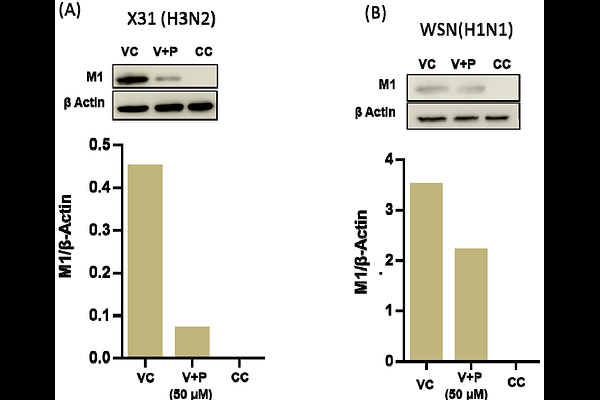
AbstractMembrane fusion is a crucial step in the infection cycle of an enveloped virus, and the development of fusion inhibitors could lead to broad-spectrum antivirals beyond the one-bug-one-drug paradigm. In our continued effort to design peptide-based fusion inhibitors that block fusion by modulating membrane physical properties rather than targeting viral proteins, we have designed a tryptophan-aspartic acid (WD) containing peptide analog, mGG21, from coronin 1. Coronin 1 has been implicated in preventing the fusion of live mycobacteria containing phagosomes with lysosomes. The mGG-21 displays around 60% inhibition in fusion pore formation (complete fusion) in model membranes by increasing the acyl chain ordering of the membrane, regardless of the cholesterol content of the membrane, unlike previously designed predecessors with 20-30 % inhibition activity. Further, we show that mGG-21 inhibits Influenza and Chikungunya virus infection in cellular models without exerting any toxicity. Taken together, our findings underscore the importance of WD repeats in designing broad-range viral fusion inhibitors.