Inflammation-induced endothelial cell activation and angiogenic sprouting are downmodulated by ubiquitin-specific peptidase 20
Inflammation-induced endothelial cell activation and angiogenic sprouting are downmodulated by ubiquitin-specific peptidase 20
Roy, B.; Wu, J.-H.; Freedman, N. J.; Shenoy, S. K.
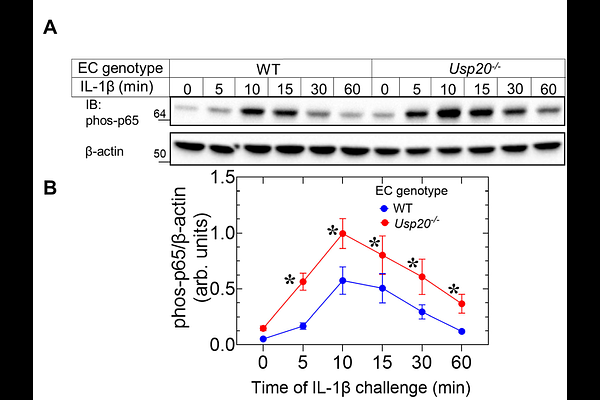
AbstractNuclear factor-{kappa}B (NF{kappa}B) mediates inflammation-driven angiogenesis, which promotes growth of atherosclerotic plaques and tumors. The deubiquitinase ubiquitin-specific peptidase 20 (USP20) suppresses NF{kappa}B activation in vascular smooth muscle cells (SMCs) and attenuates atherosclerosis, but the role of USP20 in endothelial cells (ECs) was undefined. We tested whether USP20 activity diminishes NF{kappa}B signaling in ECs and thereby diminishes angiogenesis. Cytokine-induced NF{kappa}B activity was elevated in primary ECs isolated from Usp20-/- mice as compared with ECs from wild-type (WT) mice. Similarly, cytokine-induced NF{kappa}B activity was elevated in mouse coronary endothelial cells (MCECs) expressing catalytically inactive USP20 (USP20-DN) or phospho-mimetic USP20(S334D). In contrast, cytokine stimulation of MCECs expressing WT USP20 (USP20-WT) or phospho-resistant USP20(S334A) produced blunted NF{kappa}B activity. Assessed by scratch-wound healing and spheroid assays, migration and angiogenesis of MCECs, respectively, were (a) increased by USP20-DN or USP20(S334D), and (b) decreased by USP20-WT or USP20(S334A). Angiogenesis assessed by the aortic ring assay was significantly increased in Usp20-/- mice and was suppressed by TPCA-1, an inhibitor of NF{kappa}B signaling. Angiogenesis was augmented in USP20(S334D) mouse aortic rings but reduced in USP20(S334A) mice. By screening known angiogenesis factors, we identified matrix metalloproteinase 3 (MMP3), a transcriptional target of NF{kappa}B, as a gene that is also regulated by USP20 expression and Ser334 phosphorylation. Inhibiting MMP3 reduced angiogenic sprouting in the Usp20-/- mouse aortic rings. We conclude that USP20 expression inversely correlates with the extent of angiogenesis, and that inhibiting USP20 Ser334 phosphorylation could be a useful strategy to constrain inflammation-driven angiogenesis under pathological circumstances, like cancer and atherosclerosis.