Single-Cell Multimodal Profiling Highlights Persistent Aortic Smooth Muscle Cell Changes in Diabetic Mice Despite Glycemic Control
Single-Cell Multimodal Profiling Highlights Persistent Aortic Smooth Muscle Cell Changes in Diabetic Mice Despite Glycemic Control
Tanwar, V. S.; Malek, V.; Wang, J.; Luo, Y.; Malhi, N. K.; Zhang, H.; Abdollahi, M.; Lanting, L.; Senapati, P.; Das, S.; Reddy, M. A.; Zang, C.; Miller, C. L.; Chen, Z. B.; Natarajan, R.
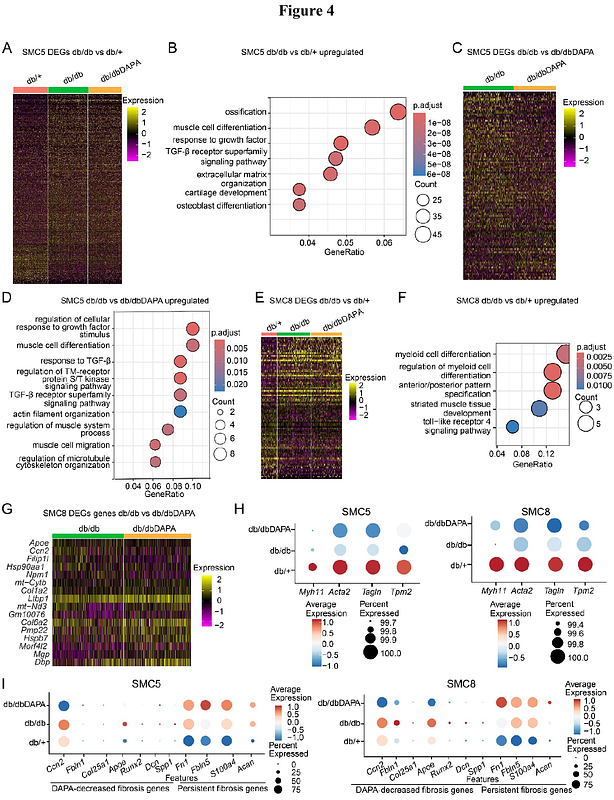
AbstractBackground: Type 2 diabetes (T2D) is associated with accelerated vascular complications like hypertension and atherosclerosis. \"Phenotypic switching\" of vascular smooth muscle cells (SMC), a major driver of these complications, is enhanced in diabetes. Despite adequate glycemic control, SMC dysfunction can persist due to \"metabolic memory\" of prior hyperglycemia. However, the mechanisms of hyperglycemic memory associated with persistent SMC dysfunction are unclear. Here, leveraging single-cell (sc) multi-omics, we examined the effect of glucose normalization on transcriptomic and epigenomic changes associated with SMC phenotypic transition in T2D mice. Methods: We treated T2D db/db mice with the antidiabetic drug dapagliflozin (DAPA) (db/dbDAPA) or vehicle (db/db), and non-diabetic control db/+ mice with vehicle for 6 weeks. Dissected aortas were subjected to scRNA-seq, scATAC-seq, and spatial transcriptomics (Xenium) to determine single-cell changes in gene expression and chromatin accessibility. Results: DAPA treatment conferred effective glycemic control in db/db mice, with significant reductions in blood glucose/hemoglobin A1c. scRNA and scATAC-seq analysis of aortas identified major cell populations, including SMC, fibroblasts, endothelial and immune cells. SMC were further clustered into 9 subtypes, including contractile and fibromyocyte-like cells. Cell composition analysis revealed decreases in contractile SMC and increases in vascular remodeling associated fibromyocyte-like SMC in db/db versus db/+ mice. Interestingly, DAPA did not reverse diabetes-induced decreases in contractile markers but reversed changes in several fibromyocyte markers in db/db mice. Pseudotime trajectory analysis revealed increased activities of fibromyocyte enriched transcription factors (TFs) during contractile to fibromyocyte transition. Furthermore, increased expression of TFs regulating fibromyocyte phenotype (e.g. Atf4, Bach1, Hand2, Fosl2) in db/db were partially reversed by DAPA, whereas reduced contractile TF (Mef2c) expression was unchanged. Spatial transcriptomics analysis further mapped aortic cell types within intact aortas and confirmed that DAPA reversed alterations in key fibromyocyte but not contractile genes in db/db mouse aortas. Conclusions: Persistent epigenetic changes may contribute to sustained vascular remodeling and dysfunction in T2D. T2D reduced contractile SMC gene expression and related chromatin accessibility and promoted phenotypic transition to fibromyocytes. These changes are only partially reversed by a widely used antidiabetic drug like DAPA, underscoring the need for more effective therapies that target hyperglycemic memory.