Dynamic DnaA-DnaB interactions at oriC coordinate the loading and coupled translocation of two DnaB helicases for bidirectional replication
Dynamic DnaA-DnaB interactions at oriC coordinate the loading and coupled translocation of two DnaB helicases for bidirectional replication
Tsuruda, T.; Yoshida, R.; Hayashi, C.; Kasho, K.; Ozaki, S.; Katayama, T.
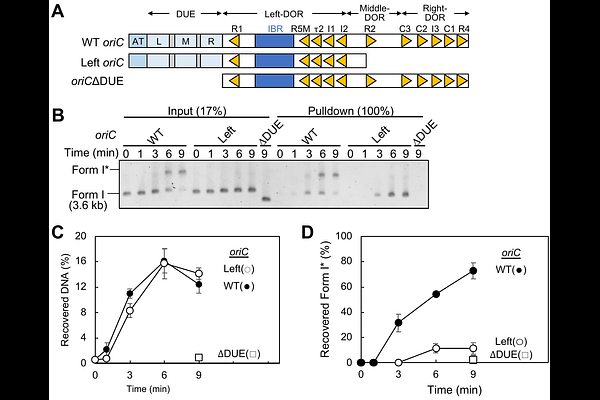
AbstractBidirectional replication is a conserved principle requiring the coordinated loading and activation of two replicative helicases at the origin. In Escherichia coli, the initiator protein DnaA constructs a higher-order initiation complex at the origin oriC which locally unwinds the DNA, and recruits DnaB helicase-DnaC loader complexes to the unwound region. We previously demonstrated that the two DnaA subcomplexes formed on oriC bind a specific DNA strand of the unwound origin, and tether individual DnaB-DnaC complexes via stable interactions between DnaA domain I and DnaB. A low-affinity DnaA-DnaB interaction mediated by DnaA domain III His136 is essential for DnaB-dependent origin unwinding. Here, we identified DnaB Thr86 as the critical residue mediating this low-affinity interaction. Structural modelling suggests that Thr86 is surface-exposed near the DNA entry site of DnaB. Functional analyses revealed that DnaB Thr86 was specifically required for DnaB loading onto the DnaA-bound strand of the unwound oriC. Furthermore, this strand-specific DnaB loading was required for enabling translocation of the opposing DnaB helicase loaded on the DnaA-free strand. Our findings define a novel regulatory mechanism of strand-specific helicase loading, mediated by the low-affinity DnaA-DnaB interactions, which ensures the coordinated loading and translocation of DnaB helicases for bidirectional replication from oriC.