Oxidative DNA Damage Drives Apoptotic Photoreceptor Loss in NMNAT1-Associated Inherited Retinal Degeneration: A Therapeutic Opportunity
Oxidative DNA Damage Drives Apoptotic Photoreceptor Loss in NMNAT1-Associated Inherited Retinal Degeneration: A Therapeutic Opportunity
Zhang, H.; Valestil, K.; Pierce, E. A.
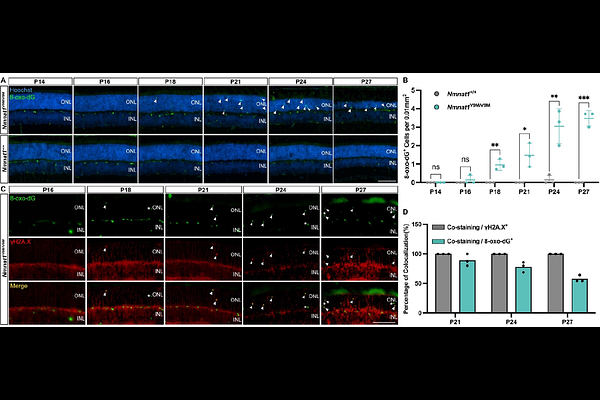
AbstractEarly-onset inherited retinal degenerations (IRDs), such as Leber congenital amaurosis (LCA) caused by pathogenic variants in the NMNAT1 gene, lead to severe vision loss in children. Despite its ubiquitous expression, reduced NMNAT1 function primarily affects photoreceptor cells (PRs) of the retina, yet the mechanisms underlying their heightened vulnerability remain incompletely understood. Here, we demonstrate that reduced NMNAT1 enzyme function due to the p.V9M mutation leads to DNA damage in PRs, characterized by the progressive accumulation of the oxidative DNA adduct 8-oxo-dG in Nmnat1V9M/V9M mutant mice. Cells with oxidative DNA damage also demonstrate DNA double-strand breaks, as evidenced by co-staining with antibodies to phosphorylated H2AX ({gamma}H2A.X). This DNA damage correlates with apoptosis-driven PR degeneration, as evidenced by caspase-9 activation and TUNEL staining in the PRs of the Nmnat1V9M/V9M mutant mice, while alternative cell death pathways such as necroptosis and parthanatos were not significantly activated. Treatment with the antioxidant N-acetylcysteine (NAC) effectively reduced oxidative DNA damage and retinal immune responses, mitigated apoptosis, and preserved cone PRs. Longitudinal assessment via optical coherence tomography (OCT) and electroretinography (ERG) revealed sustained structural and functional protection in NAC-treated mice. These findings establish oxidative DNA damage as a key driver of PR degeneration in the Nmnat1V9M/V9M model and highlight the potential of NAC as a causal gene variant-independent therapeutic strategy for NMNAT1-associated IRD and potentially other IRDs in which oxidative DNA damage contributes to disease pathogenesis.