Impact of Peptide Initial Configuration and Membrane Composition on Melittin's Pore-Forming Ability under Unbiased All-Atom Molecular Dynamics Simulations
Impact of Peptide Initial Configuration and Membrane Composition on Melittin's Pore-Forming Ability under Unbiased All-Atom Molecular Dynamics Simulations
Zhao, Z.; Guo, Y.; Cai, J.; Xie, P.; Guan, J.; Yao, L.; Liu, Y.; Chung, C.-R.; Lee, T.-Y.; Chiang, Y.-C.
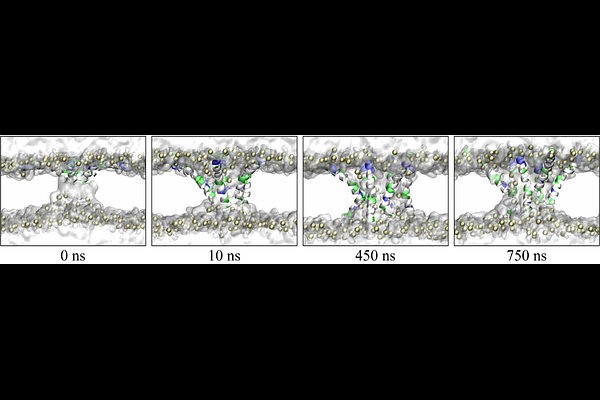
AbstractThe rising challenge of antimicrobial resistance has accelerated the search for alternative therapeutics. Antimicrobial peptides (AMPs), a class of naturally occurring defense molecules found across diverse species, are promising candidates. Despite their potent membrane-disrupting activity, the atomic details of the pore formation process remain insufficiently understood. In this study, we employed all-atom molecular dynamics (MD) simulations to investigate the pore formation process of melittin under different initial configurations. Simulations were conducted using three different membrane systems: a pure POPC bilayer, a mammalian membrane model (DOPC:Cholesterol, 9:1), and a bacterial membrane model (DOPE:DOPG, 3:1). For each system, we examined four different starting configurations, in which six melittin peptides were arranged in a star-like pattern. Our results demonstrated that the pore formation process strongly depends on the initial peptide configuration. In one specific initial arrangement (termed as Conf.I), pore formation consistently occurred within 100 nanoseconds, regardless of membrane composition. Furthermore, the simulations revealed that pore formation was more challenging in the mammalian membrane model and even more so in the bacterial membrane model, in comparison with the pure POPC bilayer. These findings are in line with previously reported minimum inhibitory concentration (MIC) and the 50% hemolysis concentration (HC50) of melittin in the literature. Additionally, we identified lysine-7 (K7) as the key residue in determining whether a stable pore can form. In configurations where the K7 side chain formed electrostatic interactions with the phosphate group of a lipid, melittin were anchored to the membrane surface, thereby preventing pore formation. In contrast, simulations of melittin mutants K7A and K7Q showed no such anchoring effect, and thus pore formation was possible in multiple initial configurations. Notably, the K7Q mutation showed a preference for pore formation in bacterial membranes over mammalian membranes, suggesting that reducing toxicity while maintaining antimicrobial efficacy is possible.