Development of a liposomal formulation of a TKI: the active loading of erlotinib.HCl
Development of a liposomal formulation of a TKI: the active loading of erlotinib.HCl
Schelker, C.; Trap, V.; Boechard, G.; Nowak-Sliwinska, P.
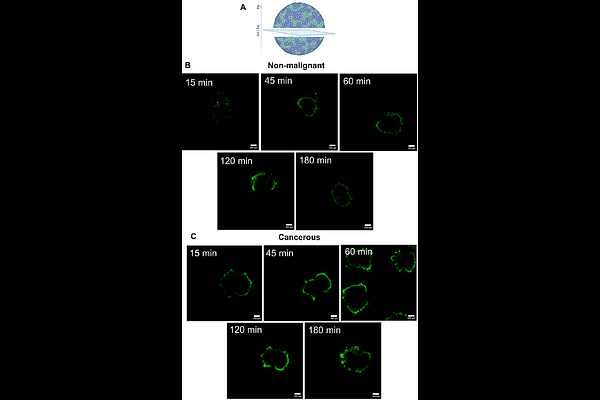
AbstractErlotinib.HCl is a tyrosine kinase inhibitor that is used to treat NSCLC and pancreatic cancer in combination with gemcitabine. It is associated with severe adverse effects. This has led researchers to explore encapsulation strategies to reduce these effects. At the same time, previous studies have investigated diverse forms of nanoparticle-based delivery systems for erlotinib.HCl, active loading into liposomes remains unexplored. In this study, we report the first successful active loading of erlotinib.HCl into liposomes. A critical step in nanomedicine development is its translation to clinical settings. Current studies typically progress from 2D cell culture to mouse models. The former lacks complexity, while the latter lacks relevant human physiology. We believe there is a gap between the two models. Therefore, we evaluated our formulation using both traditional cell lines and patient-derived organoids (PDOs). Our findings demonstrate successful active loading of erlotinib.HCl into liposomes. Comparative analysis revealed significant differences in both toxicity profiles and cellular uptake between 2D cell lines and PDOs, highlighting the importance of using complementary model systems in nanomedicine development.