Primary CD34+ cells of patients with VEXAS syndrome are highly sensitive to targeted treatment with TAK-243 and pevonedistat
Primary CD34+ cells of patients with VEXAS syndrome are highly sensitive to targeted treatment with TAK-243 and pevonedistat
Nowak, D.; Demmerle, M.; Klär, A.; Streuer, A.; Riabov, V.; Altrock, E.; Rapp, F.; Belzer, A.-C.; Klink, T.; Hahn, M.; Hofmann, F.; Nowak, V.; Weimer, N.; Obländer, J.; Palme, I.; Göl, M.; Darwich, A.; Steiner, L.; Abba, M.; Metzgeroth, G.; Hofmann, W.-K.; Schmitt, N.
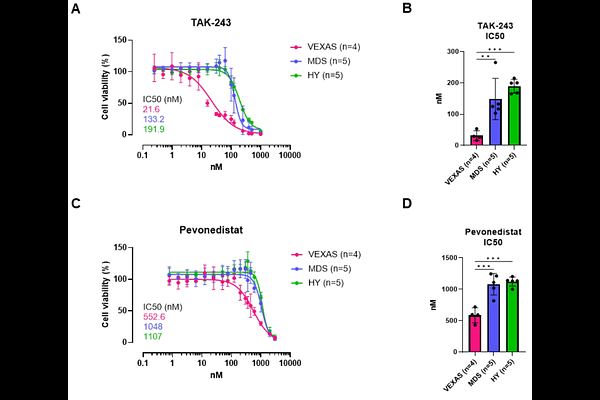
AbstractVacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic (VEXAS) syndrome is an autoinflammatory disease characterized by somatically acquired UBA1 mutations in hematopoietic stem cells. VEXAS patients present with significant therapeutic challenges such as severe treatment-resistant inflammation and predisposition to myeloid malignancies. In this study, we evaluated the efficacy of TAK-243, a UBA1 inhibitor, and pevonedistat, a NEDD8 inhibitor, in vitro on primary CD34+ hematopoietic stem and progenitor cells (HSPCs) from patients with VEXAS syndrome (n=5), myelodysplastic neoplasms (MDS) (n=10), as well as healthy controls (n=10). Our findings revealed that VEXAS patient-derived HSPCs exhibited significantly higher sensitivity to both TAK-243 (IC50: 22 nM) and pevonedistat (IC50: 553 nM) compared to MDS (IC50: 133 nM and 1048 nM, respectively) and healthy controls (IC50: 192 nM and 1107 nM, respectively) as demonstrated by reduced cell viability. Furthermore, VEXAS CD34+ cells exhibited significantly higher levels of apoptosis after treatment with both inhibitors. These results suggest that TAK-243 and pevonedistat selectively target UBA1-mutated progenitor cells, and indicate a broad therapeutic window in which malignant cells are eradicated but healthy hematopoiesis is not yet compromised. This study provides a strong rationale for further clinical investigation of TAK-243 and pevonedistat as targeted therapies for VEXAS patients, offering hope for improved management of this clinically challenging disorder and contributing to our understanding of its underlying molecular mechanisms.