A Cyclic Arginine Adduct Eclipses Carboxymethylation as the Primary Glyoxal-Derived Advanced Glycation End-Product
A Cyclic Arginine Adduct Eclipses Carboxymethylation as the Primary Glyoxal-Derived Advanced Glycation End-Product
Brutus, M. E.; Girard, C. S.; Jacob-Dolan, J. W.; Scheck, R. A.
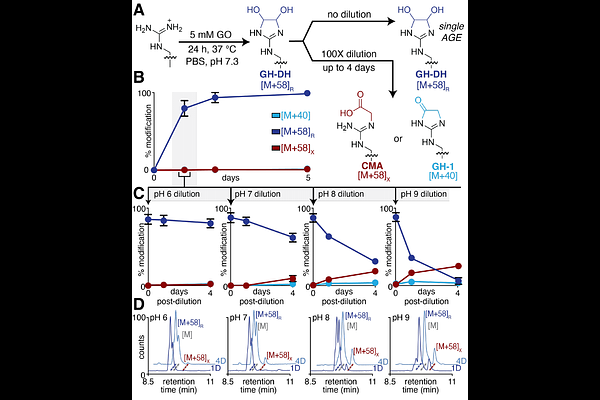
AbstractGlyoxal (GO) is a highly reactive 1,2 dialdehyde implicated in the formation of a set of disease-associated post-translational modifications (PTMs) known as advanced glycation end products (AGEs). While GO has been widely reported to modify lysine to form the highly studied AGE carboxymethyllysine (CML), here we demonstrate that GO alone leads to highly chemoselective arginine glycation, yielding a stable glyoxal-derived hydroimidazolidine (GH-DH) product. This near-exclusively formed AGE has the same mass change as carboxymethylarginine (CMA), which implies that it may have been overlooked or misattributed in prior studies. In contrast, lysine modification by GO is highly dependent on the presence of hydride-based reducing agents, suggesting that prior reports may have artifactually generated CML through pervasive use of reductive amination protocols during sample preparation. These findings challenge the standing assumptions about the landscape of GO-derived glycation, emphasizing the importance of carefully considering the impact of experimental conditions in glycation studies. By redefining the complement of GO-derived AGEs, this study provides essential new information that is greatly needed for uncovering their biology, creating new tools for their study, and discovering therapies that ameliorate or mitigate their glycation-related damage.