Allosteric modulation of dimeric GPR3 by ligands in the dimerization interface
Allosteric modulation of dimeric GPR3 by ligands in the dimerization interface
Qiu, Z.; Wang, W.; Nie, Y.; Lin, J.; Zhang, B.; Xing, H.; Zheng, S.
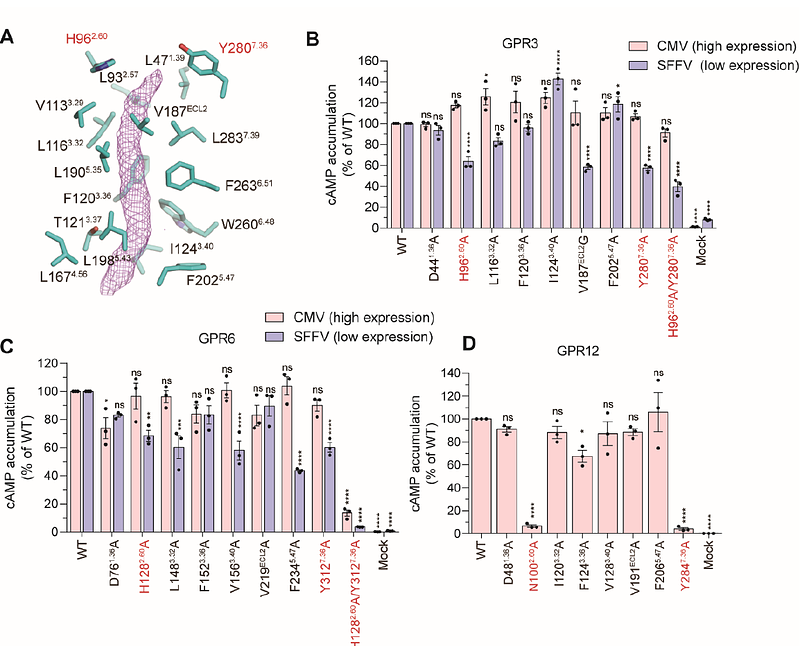
AbstractClass C G-protein coupled receptors (GPCRs) are widely recognized to function as a dimer, whereas the existence, molecular mechanisms and functional importance of dimerization in class A GPCRs remain poorly explored. GPR3, GPR6 and GPR12 are closely related to cannabinoid receptors, and are associated with neurogenerative diseases such as Parkinsons and Alzheimers diseases. Here, we determined cryo-EM structures of GPR3-Gs complex in the active state and GPR3 alone in the inactive state. We observe that GPR3 forms symmetric dimers in both states, with the dimerization interface primarily involving TM5 and TM6. Upon activation, GPR3 undergoes an outward movement of TM6 and an inward movement of TM7, alongside stabilization of helix 8, which is disordered in the inactive state. Functionally, GPR3 dimerization attenuates Gs signaling. Furthermore, we identify AF64394 as a negative allosteric modulator that targets the GPR3 dimerization interface and allosterically shifts the -helical structure of ICL2 into a random coil structure, thereby impairing Gs coupling. Our findings provide structural and mechanistic insights into class A GPCR dimerization, offering potential therapeutic strategies targeting the dimerization interface of these receptors.