Septin7 is essential in early hematopoiesis, but redundant at later stages
Septin7 is essential in early hematopoiesis, but redundant at later stages
Ronkina, N.; Verheyden, N.; Gambhir, P.; Laass, K.; Yakovleva, T.; Doerrie, A.; Abbey, M.; Menon, M. B.; Selich, A.; Galla, M.; Rothe, M.; Schambach, A.; Krueger, A.; Kotlyarov, A.; Gaestel, M.
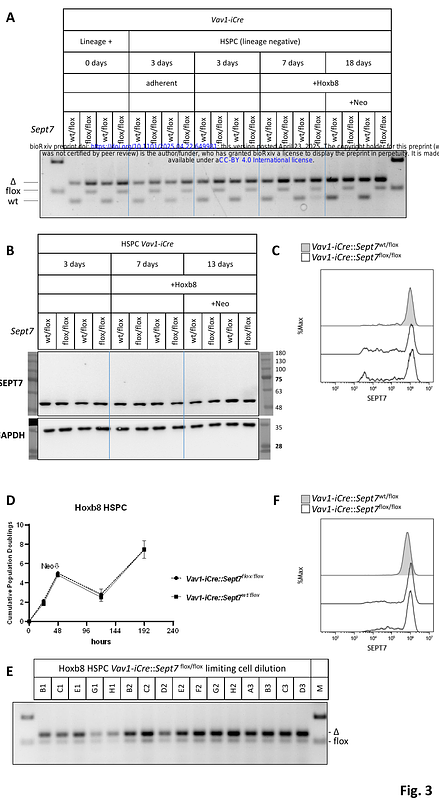
AbstractThe unique cytoskeletal protein Septin7 is generally considered to be required for cytokinesis in yeast and mammals. Whole body genetic ablation of Septin7 in mice is embryonic lethal. Septin7-deficient fibroblasts and HeLa cells are defective in cytokinesis and undergo obligate multinucleation. Unexpectedly, lymphocyte- and myeloid-specific targeting of Septin7 in mice revealed no abnormalities in blood lineage development, suggesting that Septin7 is not required for hematopoiesis. To reconcile these contradictory findings, we analyzed the effects of Septin7 deletion in hematopoietic cells from mice with either pan-hematopoietic or lymphoid lineage-specific Septin7 deletion. Our results demonstrate that Cre-induced recombination of the Septin7 floxed allele is significantly more efficient at later stages of hematopoiesis than at earlier ones, suggesting strong selection pressure against Septin7 deficiency during early hematopoiesis. In contrast, deletion of Septin7 at the common lymphoid progenitor stage, as well as in Hoxb8-immortalized hematopoietic progenitors, resulted in complete Septin7 knockouts without noticeable defects in cell division. Taken together, our findings indicate that Septin7 is essential for early hematopoiesis yet remains redundant at later stages.