Detection of a hybrid PrPfr state in the dark reversion of abathy phytochrome indicates inter-dimer allostery.
Detection of a hybrid PrPfr state in the dark reversion of abathy phytochrome indicates inter-dimer allostery.
Prodhan, S.; Meszaros, P.; Bodizs, S. A.; Zhou, Y.; Maj, M.; Westenhoff, S.
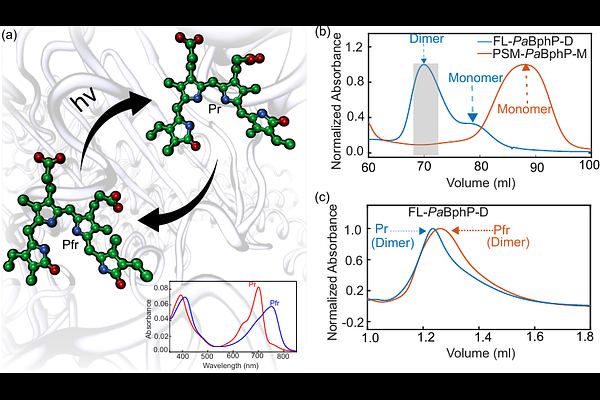
AbstractPhytochromes are photosensor proteins which detect light in plants, fungi, and bacteria. They photoswitch between red light absorbing (Pr) and far-red light absorbing (Pfr) states, however, thermal reversion in the dark is an equally important factor in controlling their signaling levels. Phytochromes are generally dimeric proteins, and mixed PrPfr states are therefore possible. These states have been implied in the dark reversion studies of plant phytochromes, but not in bacterial phytochromes. Here, we investigate the dark reversion kinetics of the bathy phytochrome from P. aeruginosa (PaBphP) using UV-Vis absorption spectroscopy. A single set of time-resolved spectra does not conclusively reveal the presence of a mixed PrPfr state, as both a direct Pr [->] Pfr model or a sequential Pr [->] PrPfr [->] Pfr model fit the spectral kinetics. However, a systematic analysis of dark reversion kinetics with varying Pr/Pfr ratios can only be satisfactorily fit by the sequential model, which indicates the presence of an intermediate PrPfr state. A newly designed monomeric variant of PaBphP provides strong support for this interpretation. Temperature-dependent kinetics revealed similarly low activation energies for the dark reversion processes of both proteins, consistent with a previously proposed keto-enol tautomerization preceding dark reversion. Interestingly, our results suggest allosteric regulation of dark reversion across the dimer, which we propose to be a contributing factor in phytochrome signaling.