Hydrogen-Induced Calcium Influx via the TRPC4-TRPC4AP Axis
Hydrogen-Induced Calcium Influx via the TRPC4-TRPC4AP Axis
Zhao, P.; li, H.; Cai, Z.; Zhang, X.; Wen, X.; Liu, Z.; Jiang, S.; Dang, Z.; Jiang, X.; Wang, J.; Liu, M.; Xie, F.; Ma, X.
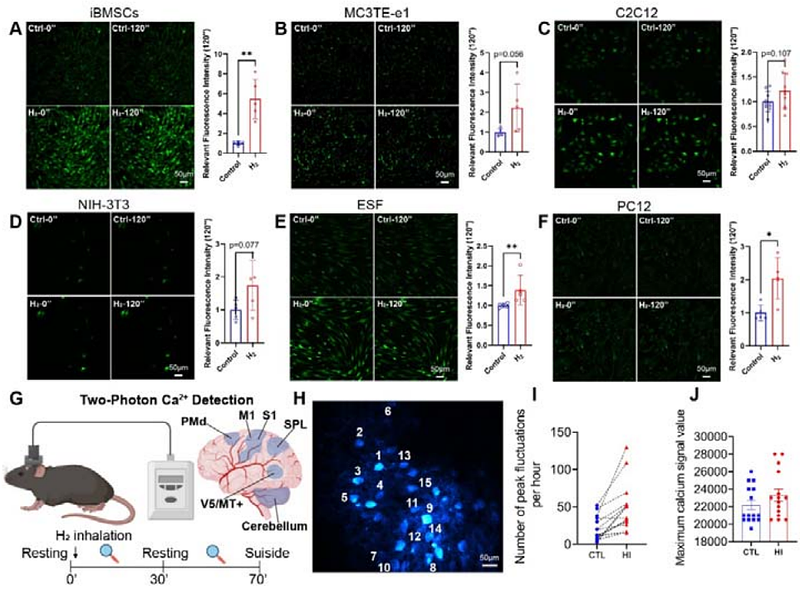
AbstractAbstract Background: Calcium ions (Ca2+) serve as universal intracellular messengers regulating diverse physiological processes, while dysregulated Ca2+ homeostasis triggers cytotoxicity. Molecular hydrogen (H2) exhibits protective effects against oxidative stress-related pathologies, but its mechanism of action remains incompletely understood. Transient receptor potential canonical 4 (TRPC4) channels and their associated protein TRPC4AP are critical mediators of Ca2+ influx ([Ca2+]i), yet their role in H2-mediated calcium signaling is unexplored. This study investigates the molecular mechanism by which H2 modulates Ca2+ dynamics through the TRPC4-TRPC4AP axis, aiming to establish its therapeutic potential for calcium-related disorders. Methods: The study employed heterogeneous cellular models (e.g., mesenchymal stem cells, neurons, fibroblasts) and in vivo two-photon calcium imaging in C57BL/6J mice. Techniques included CRISPR-Cas9 knockout, siRNA-mediated gene silencing, molecular docking (AlphaFold 3), and protein-protein interaction analysis. Calcium flux was quantified via fluorescence imaging, while mitochondrial integrity and cytoskeletal dynamics were assessed using JC-1 staining, ATPase activity assays, and live-cell imaging. Structural validation of TRPC4-TRPC4AP binding sites utilized mutagenesis and complementation experiments. Results: H2 selectively enhanced extracellular Ca2+ influx via TRPC4-TRPC4AP, with no cytotoxicity or mitochondrial dysfunction observed. Key arginine residues (730Arg-731Arg) in the TRPC4 CIRB domain formed hydrogen-bond networks essential for channel activation. In vivo, H2 increased neuronal Ca2+ transient frequency and amplitude in the primary motor cortex. TRPC4AP knockout abolished H2-induced Ca2+ influx, while mutagenesis of 730Arg/731Arg disrupted channel activity. H2 also promoted cytoskeletal remodeling and cell motility, dependent on TRPC4AP-mediated Ca2+ signaling. Conclusions: This study identifies H2 as a novel calcium agonist that activates the TRPC4-TRPC4AP axis to regulate extracellular Ca2+ influx. The 730Arg-731Arg motif in TRPC4 serves as a critical H2-sensitive site, enabling dynamic calcium homeostasis without overload. These findings provide a mechanistic basis for H2-based therapies targeting calcium dysregulation in neurodegenerative, inflammatory, and metabolic diseases, while highlighting TRPC4AP as a pivotal molecular switch for gasotransmitter signaling.