PNGaseL from Flavobacterium akiainvivens targets a diverse range of N-glycan structures
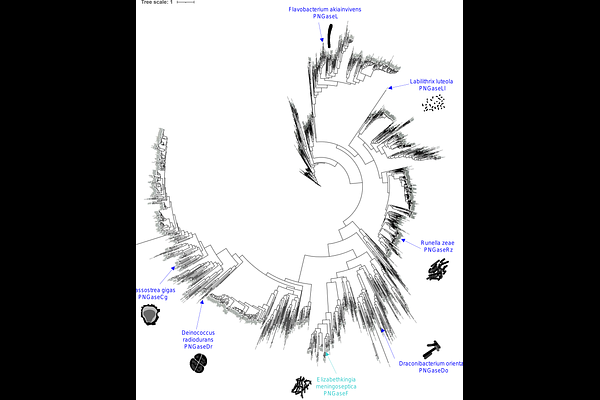
PNGaseL from Flavobacterium akiainvivens targets a diverse range of N-glycan structures
Crouch, L. I.; Bakshani, C. R.; Urbanowicz, P.; Tortosa, C. B.; Diaz, J. M. M.; Kujawska, M.; Ojuri, T. O.; Hall, L. J.; Spencer, D. I.; Bolam, D. N.
AbstractPNGases are used by a wide range of organisms to remove N-glycan structures from proteins for use as either nutrients or in glycoprotein processing. PNGaseF is the most well-characterised enzyme of this family and is widely used in glycobiology to allow study of the N-glycome of a specific protein, cell and tissues, for instance. Despite this, PNGaseF has limitations in the types of N-glycan structures it can target. In this study, we explored the specificities of six uncharacterised PNGases selected from diverse parts of the PNGaseF superfamily. One of these enzymes, PNGaseL from Flavobacterium akiainvivens, is the highlight of this study due to its very broad specificity exemplified by its ability to cleave mammalian-, plant- and invertebrate-type complex N-glycans as well as high-mannose N-glycans. A detailed biochemical and structural characterisation was carried out against a variety of substrates to illustrate the advanced capability of PNGaseL in comparison to the canonical PNGaseF and PNGaseA enzymes. To determine the optimal reaction conditions, assess stability and define limitations of PNGaseL, a series of validation studies was performed. The data reveal that PNGaseL has potential utility in a range of glycobiology applications that are superior to the current commercially available options.