Lipid dependence of connexin-32 gap junction channel conformations
Lipid dependence of connexin-32 gap junction channel conformations
Lavriha, P.; Fluri, C.; Gonzalez, J. E. H.; Korkhov, V. M.
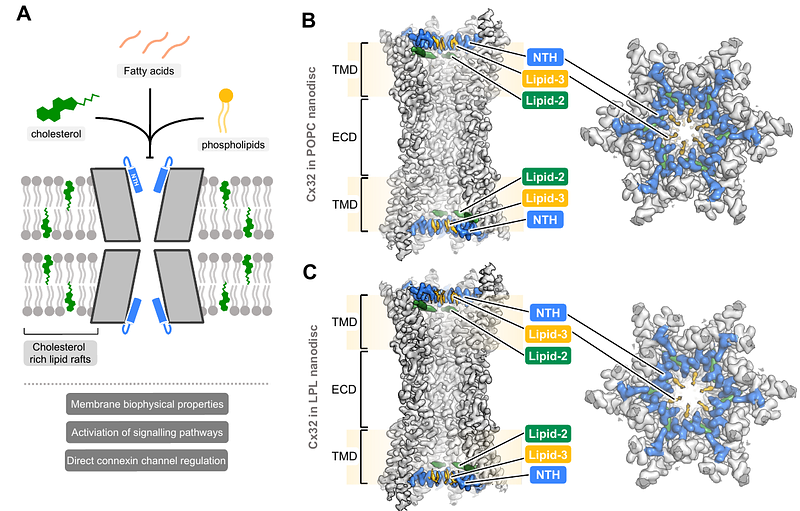
AbstractConnexin gap junction channels (GJCs) are a family of proteins that connect the cytoplasms of two neighbouring cells and allow direct intercellular exchange of solutes smaller than ~1 kDa, ensuring metabolic and electrical coupling of tissues. Connexin-32 (Cx32) GJCs mediate intercellular coupling in a variety of tissues, and specifically in the myelinating Schwann cells. Mutations in Cx32, such as W3S, are associated with peripheral neuropathy, X-linked Charcot-Marie-Tooth (CMT1X) disease. Phospholipids and sterols have been shown to regulate permeation through Cx32 GJCs, although the mechanism of regulation of Cx32 and other GJCs by lipids remains poorly understood. Here, we determine the cryo-EM structures of Cx32 GJCs reconstituted in nanodiscs, revealing that phospholipids block the pore of Cx32 GJC by binding to the site formed by N-terminal gating helices. The phospholipid-bound state is contingent on the presence of a sterol molecule bound to a hydrophobic pocket formed by the N-terminus: the N-terminal helix of Cx32 fails to sustain a phospholipid binding site in the absence of cholesterol hemisuccinate. Consistent with this, the CMT1X-linked W3S mutant which has an impaired sterol binding site adopts a conformation of the N-terminus incompatible with phospholipid binding. Our results indicate that different lipid species control connexin channel gating directly by influencing the conformation of the N-terminal gating helix.